A team of MIT chemists has recently discovered the structure of a protein, BM2, that is integral to the structure of influenza B. This protein acts as a proton channel, a membrane protein that allows protons (also called hydrogen ions or H+) to pass into the virus. Normally, these ions would be blocked by the virus’s outer membrane, called the lipid envelope. However, by lowering the pH through increasing levels of H+ ions, acidity increases and makes it easier for the virus to merge its lipid envelope with the membrane of an endosome (an intercellular compartment). After injecting its genetic content, the virus hijacks intercellular systems to rapidly copy and spread the virus further.
Mei Hong, MIT professor of chemistry and senior author of the study, remarked, “Having the atomic-resolution structure for this protein is exactly what medical chemists and pharmaceutical scientists need to start designing small molecules that can block [the channel].” Theoretically, blocking the flow of protons through this channel will inhibit infection by making it more difficult for them to enter host cells.
There are three classes of influenza viruses that affect humans: A, B, and C. Influenza A is typically the most dangerous, and is more common during the start of the flu season. They are the only kind of flu virus known to cause global flu epidemics. Influenza A is further broken down into sub-types based on the arrangement of hemagglutinin (H) surface proteins and neuraminidase (N) enzymes. Hemagglutinin helps viruses attach to the surface of host cells and infect them, while neuraminidase is required within host cells to replicate the virus. These H and N arrangements also help to name the sub-types, labelled with H1 through H18 and N1 through N11. You may be familiar with this naming scheme already if you remember the devastating 2009 outbreak of H1N1 (“swine flu”), or the 2003 H5N1 “bird flu”.
Influenza B is typically less severe and matures slower than influenza A. It is broken down into two sub-types, based solely on the arrangement of surface hemagglutinin proteins: B/Yamagata and B/Victoria. Influenza B infects mainly seals and humans, and usually shows up later in the flu season, around March and April.
Influenza type C is rarer than types A and B, and typically only causes mild illness. It has been shown to affect both humans and pigs.
Each of the three classes produces a different version of the M2 protein. The MIT research team first set out to find the differences between the M2 proteins. One key difference is that the BM2 channel allows protons to flow in either direction, whereas influenza A’s AM2 channel only allows protons to flow inside the virus. Most studies up until now have focused on the AM2 channel because of influenza A’s prevalence. However, this year influenza B infection has contrasted previous patterns. It has been unusually dominant this winter, and since September 2019 has accounted for roughly 2/3 of all flu cases reported to the U.S. Centers for Disease Control since.
To see the structure of BM2 in detail, the researchers used nuclear magnetic resonance (NMR) spectroscopy to analyze the protein down to the atomic scale. Spectroscopy is the study of the interaction between matter and electromagnetic radiation. In this case, a machine was used to observe local magnetic fields around the nuclei of the atoms making up the BM2 protein. The researchers excited the nuclei sample with radio waves, and a signal of a certain frequency was released and picked up by an NMR detection instrument. The fields released during atomic excitation are unique and characteristic to each individual compound, so the data gave details about the structure, dynamics, and chemical environment of the molecules in the protein.
The M2 channel is made of four helices, whose alignments change slightly depending on the environmental pH. High pH (low acidity) signals the channel to close. Low pH (high acidity) signals the helices to increase their tilt and open up like a pair of scissors and allows water to enter the channel. As water floods the channel, histidine (an amino acid) grabs protons from the water and delivers them to the virion (a fancy term for a virus found outside of a host cell). Unlike the AM2 channel, the BM2 channel was found to have an extra histidine at the opposite end. The research team believes this may be why the protons can flow in either direction, but what advantage or increase in virility this may provide the virus is currently unknown.
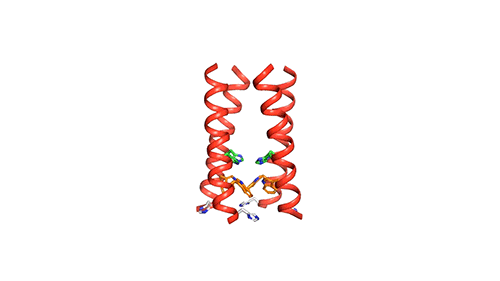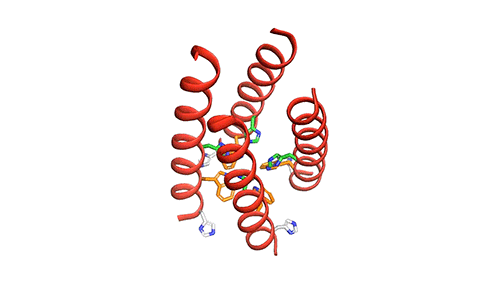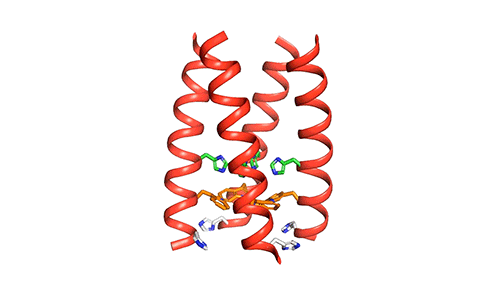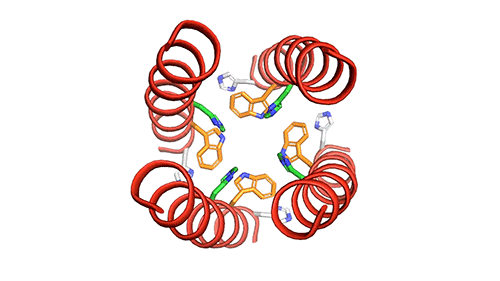
Now that a model of BM2’s structure has been created, biomedical chemists can search for ways to block it. Influenza A treatments like Amantadine and Rimantadine work by wedging into the AM2 channel and stopping the flow of protons. However, these specific drugs are ineffective at blocking proton flow in type B influenza. The precedent for this type of treatment, however, gives promise to future research.
In the meantime, we’ll have to keep reaching for tissues and Vicks Vapo-rub.
References
- Mandala VS, Loftis AR, Shcherbakov AA, Pentelute BL, Hong M. 2020. Atomic structures of closed and open influenza B M2 proton channel reveal the conduction mechanism. Nature Structural and Molecular Biology 27:160–167.
- Trafton A. 2020. Chemists unveil the structure of an influenza B protein. MIT News. Massachusetts Institute of Technology.
- Díaz FE, Dantas E, Geffner J. 2018. Unravelling the Interplay between Extracellular Acidosis and Immune Cells. Mediators of Inflammation 2018.
- Osterhaus ADME, Rimmelzwaan GF, Martina BEE, Bestebroer TM, Fouchier RAM. 2000. Influenza B Virus in Seals. Science 288:1051–1053.
- Becker ED. 1993. A Brief History of Nuclear Magnetic Resonance. Analytical Chemistry 65:295A–302A.